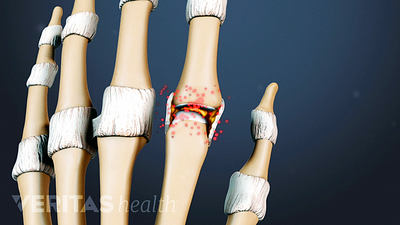
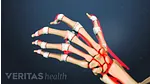
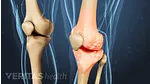
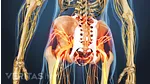
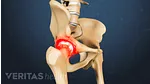
Find out why women are more likely than men to be diagnosed with the most common form of arthritis, osteoarthritis. Most arthritic joint pain, such as hip, knee, and hand pain, is caused by osteoarthritis.
Arthritis-health Blog
Rheumatoid arthritis is often linked with debilitating chronic fatigue. Experts have some ideas about how these two conditions are connected.
The recommendation to get regular exercise if you have rheumatoid arthritis (RA) is backed up by plenty of scientific research. Experts say there are at least 4 ways exercise may help ease the signs and symptoms of RA.
The inflammation caused by rheumatoid arthritis can lead to unexpected symptoms that affect vision, hearing, cardiovascular health, breathing, oral health, and more. Learn what to look out for and how to keep symptoms in check.
These 15 tips can help you cope with the joint pain and swelling, fatigue, and stress and anxiety that often come with a rheumatoid arthritis flare-up.
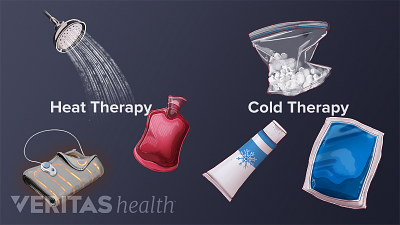
Applying a cold or hot pack to a joint can help relieve rheumatoid arthritis pain, swelling and stiffness. Learn when to use cold, when to use heat, and why these simple home treatments work.
Research shows good oral health can help relieve rheumatoid arthritis symptoms. Here are 10 ways to improve your oral hygiene so you can enjoy a healthy smile and reduce RA joint pain.
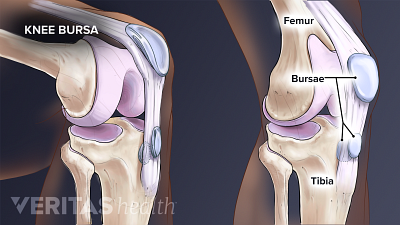
Bursitis tends to affect some joints more than others, and can even cause different symptoms depending on which bursa in a joint is affected. Learn about which joints and which bursae are most affected by bursitis.