Physicians typically prescribe topical medications to patients who
- Do not get satisfactory pain relief from over-the-counter medications
- Have sensitivities to oral medications
- Have stomach ulcers or other conditions that can cause gastrointestinal bleeding
If over-the-counter medications have failed, topical medication for arthritis relief may be considered.
In the U.S., prescription topical arthritis pain medications currently include:
- Topical NSAIDs
- Certain lidocaine products, such as 5% lidocaine patches
Like all prescription medication, topical pain relief products are potentially toxic. Patches should be kept away from pets and children and folded and disposed of properly after use.
In This Article:
- Topical Pain Relief for Arthritis
- Over-the-Counter Topical Arthritis Pain Relief
- Prescription Topical Arthritis Pain Relief
- Uncommon Prescription Topical Analgesics for Arthritis Pain
Prescription Topical NSAIDs
Research evidence suggests topical non-steroidal anti-inflammatory drugs (NSAIDs) reduce arthritis pain as well as oral NSAIDs. 1 Zhang Y, Shi S, Chen X, Peng M. Functionalized magnetic nanoparticles coupled with mass spectrometry for screening and identification of cyclooxygenase-1 inhibitors from natural products. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Jun 1;960:126-32. doi: 10.1016/j.jchromb.2014.04.032. Epub 2014 Apr 26. PubMed PMID: 24793085. , 2 Derry S, Moore RA, Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2012 Sep 12;9:CD007400. doi: 10.1002/14651858.CD007400.pub2. Review. PubMed PMID: 22972108; PubMed Central PMCID: PMC4160008. In the U.S., topical NSAIDs for arthritis pain are made with an active ingredient called diclofenac.
Two commonly prescribed diclofenac products are:
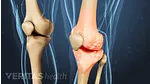
- Voltaren Gel, which is FDA-approved to treat pain caused by osteoarthritis in the knees, ankles, feet, elbows, wrists, and hands.
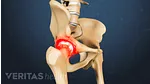
- Pennsaid topical solution, which is FDA-approved to treat pain caused by knee osteoarthritis
Neither product is formally approved for use on deeper joints, such as the shoulder, hip, and low back.
Topical diclofenac users should follow manufacturers’ instructions and carefully measure doses according to the joint being treated. Upper extremity joints (e.g. elbows) require smaller amounts than lower extremity joints (e.g. knees).
Other types of topical NSAIDs, with active ingredients such as ibuprofen or ketoprofen, may be available in Europe or special-ordered from compounding pharmacies. These products may become more widely available in the U.S. in upcoming years.
Topical NSAIDs: Potential side effects and drug interactions
The most common side effect of topical NSAIDs is dry skin at the application site.
3
Roth SH, Fuller P. Pooled safety analysis of diclofenac sodium topical solution 1.5% (w/w) in the treatment of osteoarthritis in patients aged 75 years or older. Clin Interv Aging. 2012;7:127-37. doi: 10.2147/CIA.S30884. Epub 2012 Jun 11. PubMed PMID: 22791985; PubMed Central PMCID: PMC3393357.
,
4
Simon LS, Grierson LM, Naseer Z, Bookman AA, Zev Shainhouse J. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain. 2009 Jun;143(3):238-45. doi: 10.1016/j.pain.2009.03.008. Epub 2009 Apr 19. PubMed PMID: 19380203.
Topical diclofenac medication has absorption locally, at the site of the painful joint, as well as into the bloodstream. This means topical diclofenac products:
- May pose gastrointestinal side effects, though these side effects tend to be less frequent than with oral NSAIDs. 5 Derry S, Moore RA, Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2012 Sep 12;9:CD007400. doi: 10.1002/14651858.CD007400.pub2. Review. PubMed PMID: 22972108; PubMed Central PMCID: PMC4160008.
- Interact with oral medications, such as oral NSAIDs, aspirin, anticoagulants (e.g. warfarin), and methotrexate.
- Are not ideal for people who have three or more painful joints. Applying medication to several joints can result in too much medication being absorbed through the skin. (For these people, oral medications may be more appropriate.)
In addition, topical NSAIDs may pose other potential risks. For example, the FDA warns that Voltaren Gel poses a potential risk to the liver, and advises patients to tell their doctors if they have liver problems before using diclofenac products. 6 FDA MedWatch Safety Alerts: December 2009. Food and Drug Administration. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm196683.htm Updated February 2010. Accessed November 14, 2015.
To decrease the risk of side effects and interactions, patients should tell their health care provider about medications and supplements they are currently taking.
Prescription Topical Lidocaine
Lidocaine is a local anesthetic sometimes used to treat arthritis and other musculoskeletal pain. It is sold in over-the-counter products as well as in prescription products, including ointments, gels and creams as well as adhesive patches that contain lidocaine solution.
Evidence suggests that 5% lidocaine patches help relieve arthritis pain. 7 Gammaitoni AR, Galer BS, Onawola R, Jensen MP, Argoff CE. Lidocaine patch 5% and its positive impact on pain qualities in osteoarthritis: results of a pilot 2-week, open-label study using the Neuropathic Pain Scale. Curr Med Res Opin 2004;20 Suppl 2:S13-9. PubMed PMID: 15563742. These patches, sold under the brand name Lidoderm Patch, offer some patients deep pain relief. (One limitation of all topical lidocaine products is that lidocaine does not always penetrate deep below the skin, where joint pain originates.)
Potential side effects and drug interactions
Topical lidocaine numbs the skin and should not be used while icing or heating the painful joint.
See 3 Types of Cold Packs for Arthritis
It is possible for topical lidocaine to enter the bloodstream. If too much lidocaine is absorbed through the skin, a person can experience serious side effects, such as irregular heartbeats, breathing problems, seizures, and coma. People are advised to use this medication as directed and avoid using more than necessary. For example, Lidoderm Patch users are instructed to use three or fewer patches and to spend at least 12 hours per day without any patches. 8 Lidoderm Lidocaine Patch: How Lidoderm Works. Endo Pharmaceuticals. http://www.lidoderm.com/dtc/how-lidoderm-works.aspx January 2013. Accessed November 14, 2015.
- 1 Zhang Y, Shi S, Chen X, Peng M. Functionalized magnetic nanoparticles coupled with mass spectrometry for screening and identification of cyclooxygenase-1 inhibitors from natural products. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Jun 1;960:126-32. doi: 10.1016/j.jchromb.2014.04.032. Epub 2014 Apr 26. PubMed PMID: 24793085.
- 2 Derry S, Moore RA, Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2012 Sep 12;9:CD007400. doi: 10.1002/14651858.CD007400.pub2. Review. PubMed PMID: 22972108; PubMed Central PMCID: PMC4160008.
- 3 Roth SH, Fuller P. Pooled safety analysis of diclofenac sodium topical solution 1.5% (w/w) in the treatment of osteoarthritis in patients aged 75 years or older. Clin Interv Aging. 2012;7:127-37. doi: 10.2147/CIA.S30884. Epub 2012 Jun 11. PubMed PMID: 22791985; PubMed Central PMCID: PMC3393357.
- 4 Simon LS, Grierson LM, Naseer Z, Bookman AA, Zev Shainhouse J. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain. 2009 Jun;143(3):238-45. doi: 10.1016/j.pain.2009.03.008. Epub 2009 Apr 19. PubMed PMID: 19380203.
- 5 Derry S, Moore RA, Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2012 Sep 12;9:CD007400. doi: 10.1002/14651858.CD007400.pub2. Review. PubMed PMID: 22972108; PubMed Central PMCID: PMC4160008.
- 6 FDA MedWatch Safety Alerts: December 2009. Food and Drug Administration. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm196683.htm Updated February 2010. Accessed November 14, 2015.
- 7 Gammaitoni AR, Galer BS, Onawola R, Jensen MP, Argoff CE. Lidocaine patch 5% and its positive impact on pain qualities in osteoarthritis: results of a pilot 2-week, open-label study using the Neuropathic Pain Scale. Curr Med Res Opin 2004;20 Suppl 2:S13-9. PubMed PMID: 15563742.
- 8 Lidoderm Lidocaine Patch: How Lidoderm Works. Endo Pharmaceuticals. http://www.lidoderm.com/dtc/how-lidoderm-works.aspx January 2013. Accessed November 14, 2015.